Abstract
Introduction: PNH is a hematopoietic stem cell disorder with the expansion of a PIGA mutated clone(s). PNH clones are deficient in the membrane expression of GPI-anchored proteins including CD55 and CD59, which leave erythrocytes susceptible to complement attack, resulting in intravascular hemolysis. Many symptoms experienced by PNH patients such as fatigue, dyspnea, and stomachache have been found to be associated with serum free hemoglobin (Hb) released from erythrocytes by intravascular hemolysis (JAMA. 2005;293(13):1653-1662). Eculizumab (Soliris®), a humanized monoclonal antibody against Complement Factor C5, has shown dramatic effect in blocking intravascular hemolysis. Previous reports have revealed that eculizumab has not only improved anemia but also patients' quality of life (QoL) (N Engl J Med. 2006; 355: 1233-1243, Blood. 2008; 111: 1840-1847, Int J Hematol. 2011; 93: 36-46). However, factors which may be associated with improvement of QoL have yet to be fully understood. To clarify the clinical significance of QoL improvement in PNH patients treated with eculizumab, we analyzed the change in QoL in 54 PNH patients treated with eculizumab based on the data from the post-marketing surveillance (PMS) of eculizumab in Japan.
Methods: All PNH patients treated with eculizumab have been registered in PMS based on the revised Pharmaceutical Affairs Law in Japan since June 2010. Of 491 patients registered as at March 2017, QoL questionnaires were obtained from 225 patients. 54 patients who received both baseline and one-year assessment of QoL were analyzed in this study. QoL was assessed using Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-Fatigue) and European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30) instruments. Summary statistics were obtained from the figures of QoL scoring systems and the lab values, and paired Student's t-test was used for the change of the values in one year from the baseline. Canonical correlation analysis was performed among QoL variates (consisted of 16 items) and lab data variates (LDH, Hb, and platelets).
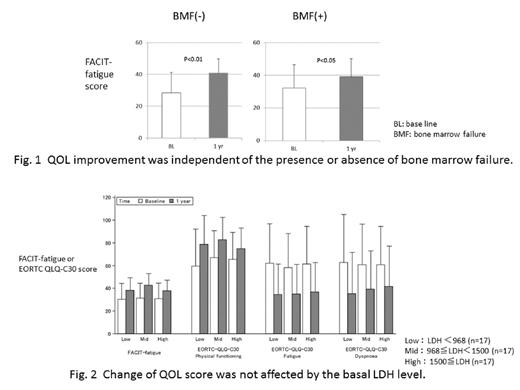
Results: There was no bias in the baseline characteristics between the total (491) and the analyzed (54) patients. One-year administration of eculizumab improved serum LDH level (mean: from 1435.3 to 289.5 U/L), Hb level (mean: from 8.22 to 9.46 g/dL), and QoL in most of their items in comparison with the baseline. In particular, significant improvement of EORTC QLQ-C30 was shown in the items of fatigue, dyspnea, physical function, and global health status. Canonical correlation analysis revealed a high correlation between QoL variates and lab data variates (Canonical R=0.8393, p=0.004). Of the lab data variates, LDH (Canonical correlation coefficient: -0.91) and Hb (0.76) showed strong correlations with QoL improvement as expected. Interestingly, QoL improvement was independent of patient's baseline characteristics such as the coexistence of aplastic anemia and/or myelodysplastic syndrome and the degree of lactate dehydrogenase (LDH) (Fig. 1, 2).
Conclusion: In this study, QoL improvement following treatment with eculizumab was statistically strongly correlated to the degree of hemolysis, which is consistent with the previous reports. Herein we also demonstrated, for the first time, that the degree of improvement was independent of the baseline serum LDH level before eculizumab treatment and of the presence or absence of bone marrow failure. Those PNH patients without eculizumab treatment due to mild hemolysis might benefit from eculizumab treatment regardless of the presence of bone marrow failure syndromes.
Ueda: Alexion Pharma GK: Honoraria, Research Funding. Obara: Alexion Pharma GK: Honoraria, Research Funding. Yonemura: Alexion Pharma GK: Honoraria, Research Funding. Noji: Alexion Pharma GK: Honoraria, Research Funding. Matsuda: Alexion Pharma G.K.: Employment. Akiyama: Alexion Pharma G.K.: Employment. Chiba: Nippon Shinyaku: Honoraria, Research Funding. Kawaguchi: Alexion Pharma G.K.: Honoraria; Novartis,Pfizer: Consultancy, Honoraria. Shichishima: Alexion Pharma GK: Honoraria. Okamoto: Bristol-Myers K.K: Honoraria; Pfizer Japan: Speakers Bureau; Alexion Pharma G.K.: Honoraria; Astellas: Honoraria; Pfizer: Research Funding; Chugai Pharmaceutical Co.: Research Funding; Novartis: Research Funding; Bristol-Myers K.K: Research Funding. Nishimura: Alexion Pharma GK: Honoraria, Research Funding. Kanakura: Fujimotoseiyaku: Research Funding; Alexion Pharmaceuticals, Inc.: Honoraria, Research Funding; Kyowa Hakko Kirin: Research Funding; Astellas: Research Funding; Eisai: Research Funding; Shionogi: Research Funding; Chugai Pharmaceutical: Research Funding; Nippon Shinyaku: Research Funding; Pfizer: Research Funding; Bristol Myers: Research Funding; Toyama Chemical: Research Funding. Ninomiya: Alexion Pharma GK: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal